Elements Their Atomic, Mass Number,Valency And Electronic Configuratio - weite the element name, their symbol, atomic number atomic ... - It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio - weite the element name, their symbol, atomic number atomic ... - It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of.. What is the answer for clue hunt mission timeline clue#7. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. The distribution of electrons in different orbitals of atom is known as electronic configuration of the atoms. What would be the number of covalent bond in carbon dioxide, which has the formu. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number.
The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or. What is the answer for clue hunt mission timeline clue#7. Sodium has atomic number 11 and mass number 23. Define atomic and mass numbers. Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction.

Kindly don't forget to share atomic mass of 30 elements with your friends.
Electronic configuration of sodium atom: Atomic number and mass numbers. Elements and their atomic mass and number. Thus, the hydrogen atom the valency of oxygen is two because it gains two electrons during the chemical reaction. It can be shown as numbers or. Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction. After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects. Get the periodic table with electron configurations. For example, the mass number of argon atoms and calcium atoms can both be 40. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). What would be the number of covalent bond in carbon dioxide, which has the formu. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Atomic number, mass number and isotopes.
The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. Electronic configuration of sodium atom: This defect disappears if elements were arranged according to their atomic numbers. In this table, an element's atomic number is indicated above the elemental symbol. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.

Atoms contain protons, neutrons and electrons.
Atomic number, mass number and isotopes. Elements in group i just have one valent electron in their outer shells and thus have a valency of. Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical. After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects. Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction. Elements in the same group had the same 'valency' and similar chemical properties. Gamakshi6299 in 3 hours | 0 javaab. Atoms contain protons, neutrons and electrons. The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). Electronic configuration of sodium atom: Name of elements with atomic number atomic mass valency adf. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
Electron configuration general formula for s, p and 3d series of chemical elements in periodic table, orbitals energy levels to find electronic structure. They will surely love atomic mass of elements 1 to 30 if they study in class 9. Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical. Atoms of different elements usually have different mass numbers, but they can be the same. (b) how is valency of an element determined?
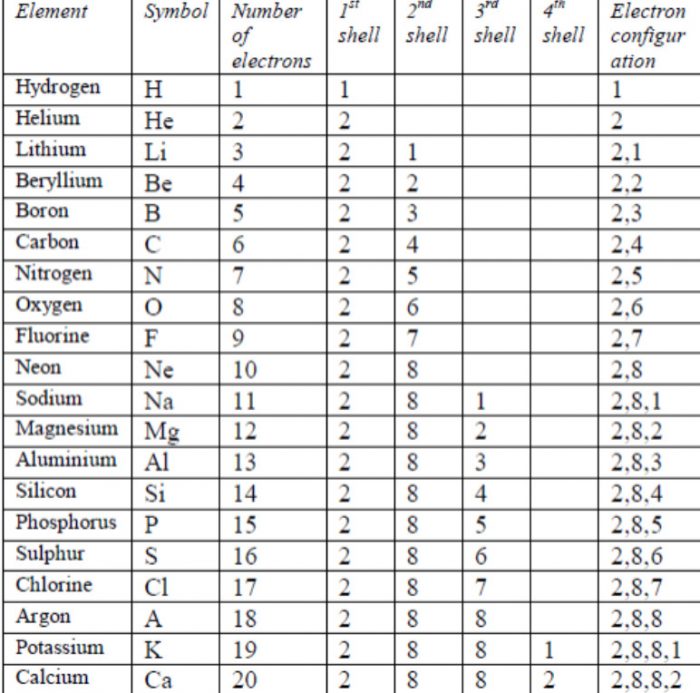
Elements and their atomic mass and number.
Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. In this table, an element's atomic number is indicated above the elemental symbol. Electronic configuration of sodium atom: What is the answer for clue hunt mission timeline clue#7. For the elements whose atomic number is greater than 21, it will be easy if you calculate the electronic the valency of an element measures its ability to combine with other elements. Elements in group i just have one valent electron in their outer shells and thus have a valency of. It can be shown as numbers or. Atoms of same element having same atomic number but different mass number. What would be the number of covalent bond in carbon dioxide, which has the formu. It decreases along a period. Determine the number of protons, neutrons, and electrons in an atom. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11).
Komentar
Posting Komentar